
Basic Violet 2


Description
Basic Violet 2 (New Fuchsin) has not only industrial and staining uses, but also a complex safety and regulatory profile according to the EU Scientific Committee on Cosmetic Products, the submitted data for its use in hair dyes was insufficient, particularly lacking a sub-chronic toxicity study, skin‑absorption data, and full genotoxicity evaluation. Toxicologically, it shows potential for eye and skin irritation and is classified as possibly carcinogenic (with chronic exposure concerns. From an environmental standpoint, as a cationic basic dye it is highly persistent, poorly biodegradable, and can pose ecotoxicity risks when released in textile wastewater. Its physico‑chemical properties include very low vapor pressure, a boiling point over 300 °C, and significant water solubility (≈10–20 g/L), making it quite stable under typical conditions.
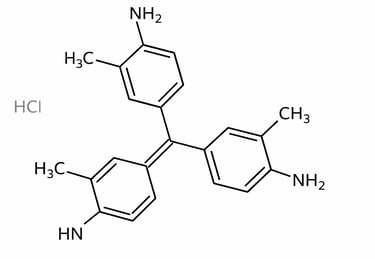
Chemical Structure of Basic Violet 2
Appearance of Basic Violet 2
Uses
1. Biological & Microbiological Staining
Packing
Export Worthy packing 5 Kg Drum, 25 Kg Drum, 50 Kg Drum.
Storage Conditions
Store in cool and dry place at room temperature, protect from direct sunlight.
Molecular Formula
CAS No.
IUPAC Name
Synonyms
Molecular Weight
Appearance
Solubility
Dye Content (Spectrophotometry)
C₂₂H₂₄ClN₃
3248‑91‑7
4-[(4‑amino-3‑methylphenyl)-(4‑imino-3‑methylcyclohexa-2,5-dien‑1‑ylidene)methyl]-2-methylaniline; hydrochloride
Triaminotriphenylmethane dye
~365.9 g/mol
Pink, Magenta, or Dark purple solid/powder
98%
:
:
:
:
:
:
:
:
Ziehl–Neelsen (Acid-Fast) Staining: One of the main uses of Basic Violet 2 is in carbol‑fuchsin solutions for acid-fast staining. Because acid-fast bacteria (like Mycobacterium) have lipid-rich, waxy cell walls, they take up the fuchsin more readily when the solution is heated, forming a stable mycolate–fuchsin complex.
Gram Staining: It is also used in Gram staining protocols. As a cationic dye, it binds strongly to negatively charged bacterial cell components, and is used in certain formulations to stain cells.
Schiff’s Reagent: Basic Violet 2 is employed in conjunction with Schiff’s reagent (in the periodic acid–Schiff process) for detecting aldehyde groups in tissue — useful in histology and pathology.
Cellular / Histological Stains: In histology and cytology, it helps highlight cellular structures (nuclei, cytoplasm) because of its cationic nature, binding to acidic cell components like nucleic acids.
2. Cosmetic Use / Hair Coloring
3. Textile & Leather Dyeing
In the textile industry, Basic Violet 2 is used to dye fabrics, giving bright, long-lasting magenta/violet shades.
It’s also used in leather processing for coloring leather materials, leveraging its strong dyeing affinity.
Soluble in DMSO, Methanol, Propylene glycol.
4. Analytical Chemistry
It’s used as a pH indicator in some analytical contexts. According to Chem‑Impex, New Fuchsin can function in chemical analyses beyond its role as a dye.
In thin-layer chromatography (TLC), Basic Violet 2 has been used as a developing agent, particularly for the detection of certain perfluorinated organics.
Basic Violet 2 is used as a direct (non-oxidative) hair dye In Europe, regulatory submissions noted its use in both semi-permanent and oxidative hair-dye formulations.
The EU’s SCCP (Scientific Committee on Cosmetic Products) has limited its concentration in cosmetics for skin-contact products, it’s allowed only up to 5 pm.
5. Forensic Science
There is research on using Basic Violet 2 for latent fingerprint (fingermark) visualization. It acts as a lipid-specific reagent, binding to sebaceous (fatty) components in fingerprints, which helps reveal ridge patterns.
6. Environmental / Research Applications
In environmental research, Basic Violet 2 is used in photocatalytic degradation studies: it serves as a model pollutant for testing the efficiency of photocatalysts (like TiO₂, ZnO, etc.) in degrading organic dyes.
It’s also used in studies of biodegradation, where microbial consortia (fungi + bacteria) are employed to break down or decolorize the dye from contaminated water.
CI No.
:
42520
Contact
Reach out for inquiries and partnerships.
Follow
Connect
© 2008-2025, All Rights reserved by MODASA Pharmaceuticals Pvt. Ltd.
